What Best Describes a Material's Ability to Dissolve
Specific gravity D. 1 Waters polarity allows it to form covalent bonds with many substances 2 Natural water has a pH of approximately 56 which is slightly acidic.

Solved For Each Multiple Choice Question Choose The Best Chegg Com
Def any mixture of 2 or more immiscible liquids in which one liquid is dispersed in the other.
. Which property describes a materials ability to dissolve. There is not a definite term for the ability of a substance to dissolve other substances. In other words it is the degree at which a solute dissolves in a solvent to form a solutionSolubility describes the ability of a substance to dissolve in a solvent at a given temperature.
Which of the following best describes waters ability to dissolve certain substances such as glucose but remain separate from other substances such as oils. 2 Show answers Another question on Biology. Solubility is a property referring to the ability for a given substance the solute to dissolve in a solvent.
Solubility Melting point Boiling point Thermal conductivity. A substances ability to dissolve in another. What term describes the ability of two or more gases or liquids to mix or dissolve into each other.
It is measured in terms of the maximum amount of solute dissolved in a solvent at equilibrium. ExplanationSolubility is the property of a substances which is the capability of a given substance that is called a solute to dissolve in a solvent such as water. The ability of something to dissolve in a liquid.
Def the substance being dissolved in the solution. Solubility is the ability of a solute to remain dissolved in water to form a solution. For example water is a polar solvent and it will dissolve salts and other polar molecules but not non-polar molecules like oil.
Which of the following best describes waters ability to dissolve certain substances such as glucose but remain separate from other substances such as oils. Punnett squares are used to show possible combinations of alleles or to predict the probability. Measures a substances ability to conduct heat across the material to another object.
How does the polarity of water contribute to its ability to dissolve so many substances. Regents Chemistry defines solubility as the maximum quantity of a solute that may be dissolved in a given quantity of solvent at a given temperature to make a saturated solution. Answer choices Water molecules are polar consisting of two partially positive hydrogen atoms and one partially negative oxygen atom.
Building glycogen from glucose molecules is an example of what. It is measured in terms of the maximum amount of solute dissolved in a solvent at. Which of the following best describes waters ability to dissolve certain substances such as glucose but remain separate from other substances such as oils.
The ability of a material to dissolve in liquid is termed a. What best describes a materials ability to dissolve. Solubility is a property referring to the ability for a given substance the solute to dissolve in a solvent.
Choice A Due to hydrogen bonding water is more dense as a liquid than a solid. Option A is correct. D Plastic has low electrical conductivity and metal has high electrical conductivity.
The resulting solution is called a saturated solution. Option B is incorrect. Def the dissolving medium.
Saturation occurs when no more solute can be dissolved in the solvent. A substances ability to transfer energy to another substance or object. Acids can dissolve more substances than bases.
Answer choices Water molecules are polar consisting of two partially positive hydrogen atoms and one partially negative oxygen atom. ExplanationSolubility is the property of a substances which is the capability of a given substance that is called a solute to dissolve in a solvent such as water. Petrol is a non-polar solvent and will dissolve oil but will not mix with water.
15 Questions Show answers. There is not a definite term for the ability of a substance to dissolve other substances. Boiling pint is the temperature at which the vapor pressure of liquid is equal to the atmospheric pressure.
A materials physical state has no effect on the behavior of its container. Any measurable or observable attribute that describes matter. The same PPE will be worn at a hazmat incident no matter the physical state of the hazardous material.
In other words it is the degree at which a solute dissolves in a solvent to form a solutionSolubility describes the ability of a substance to dissolve in a solvent at a given temperature. A substances ability to dissolve in another. Polarionic solvents dissolve polarionic solutes and non-polar solvents dissolve non-polar solutes.
Def cant dissolve any more solute under the given conditions. A physical property that describes how tightly packed molecules are in a substance.

Solved Question 7 5 5 Marks Consider The Following Chegg Com

Material Science And Engineering Flashcards Quizlet
10 5 The Solid State Of Matter Chemistry
Materials Free Full Text Thermal Stability Of Aluminum Alloys Html

Material Science And Engineering Flashcards Quizlet

Material Science And Engineering Flashcards Quizlet
Materials Free Full Text Thermal Stability Of Aluminum Alloys Html
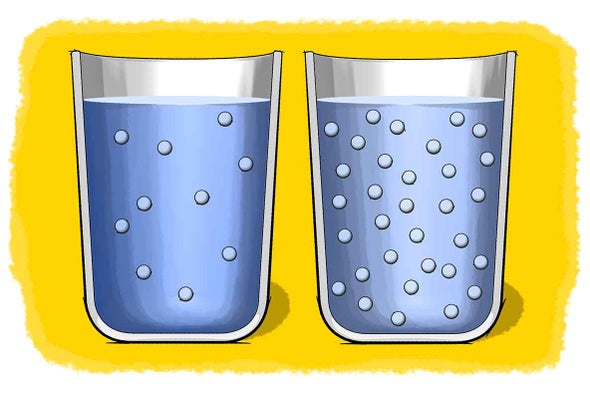
Solubility Science How Much Is Too Much News And Research Scientific American

Material Science And Engineering Flashcards Quizlet
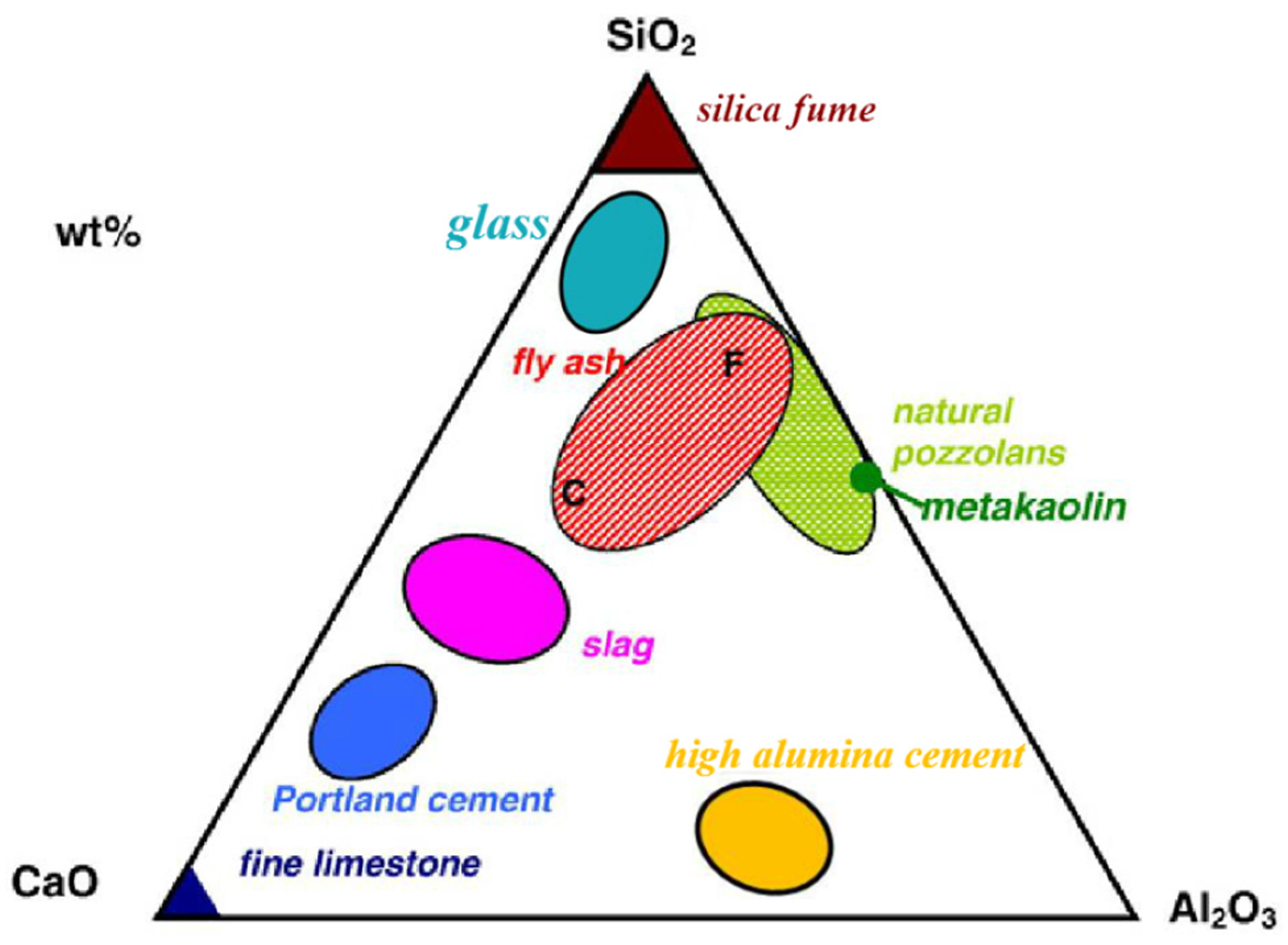
Materials Free Full Text Research Progress On Controlled Low Strength Materials Metallurgical Waste Slag As Cementitious Materials Html
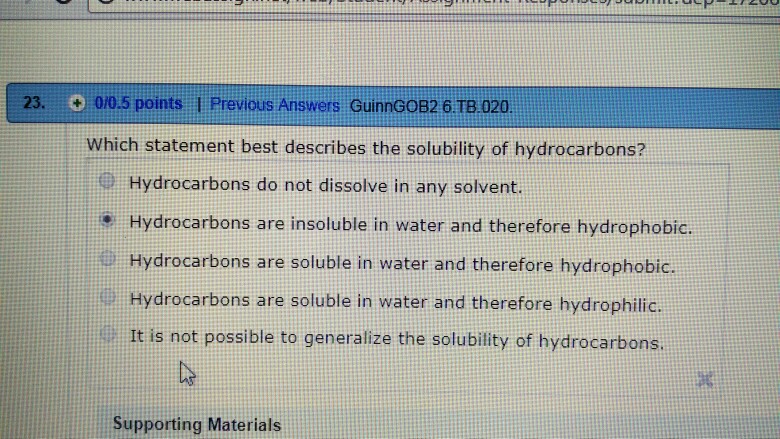
Solved 23 0 0 5 Points I Previous Answers Guinngob2 6 Chegg Com

Materials Free Full Text Thermal Stability Of Aluminum Alloys Html
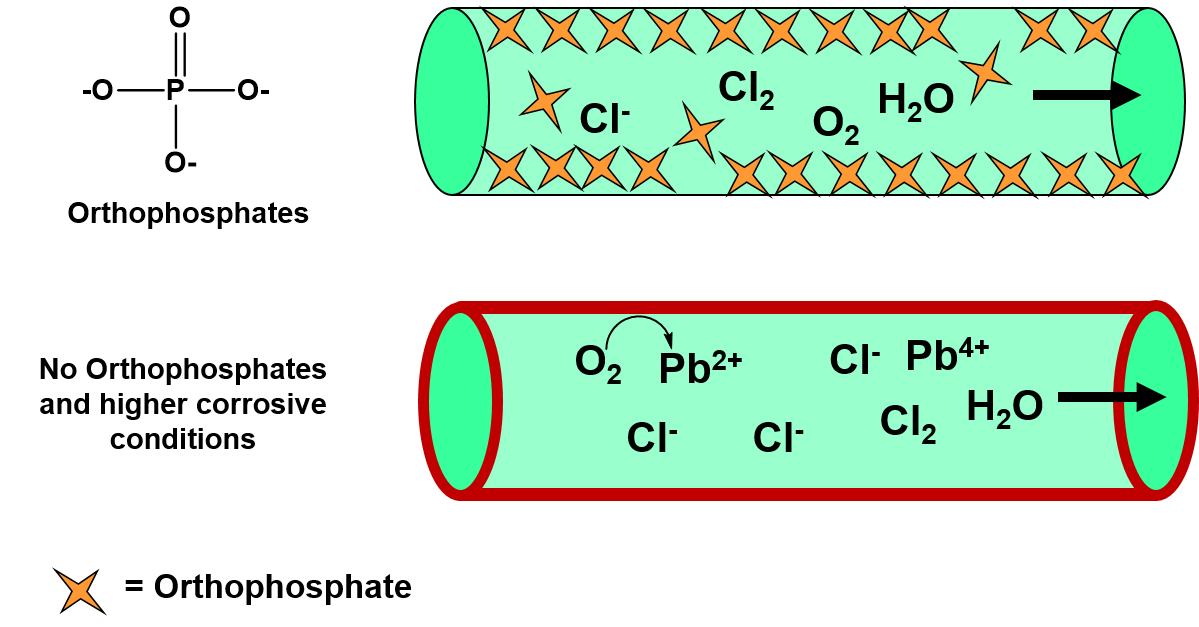
Ch104 Chapter 7 Solutions Chemistry
2 2 Water Concepts Of Biology 1st Canadian Edition

10 7 Which Characteristic Is Shared By All Prokaryotes And

Conductivity An Overview Sciencedirect Topics
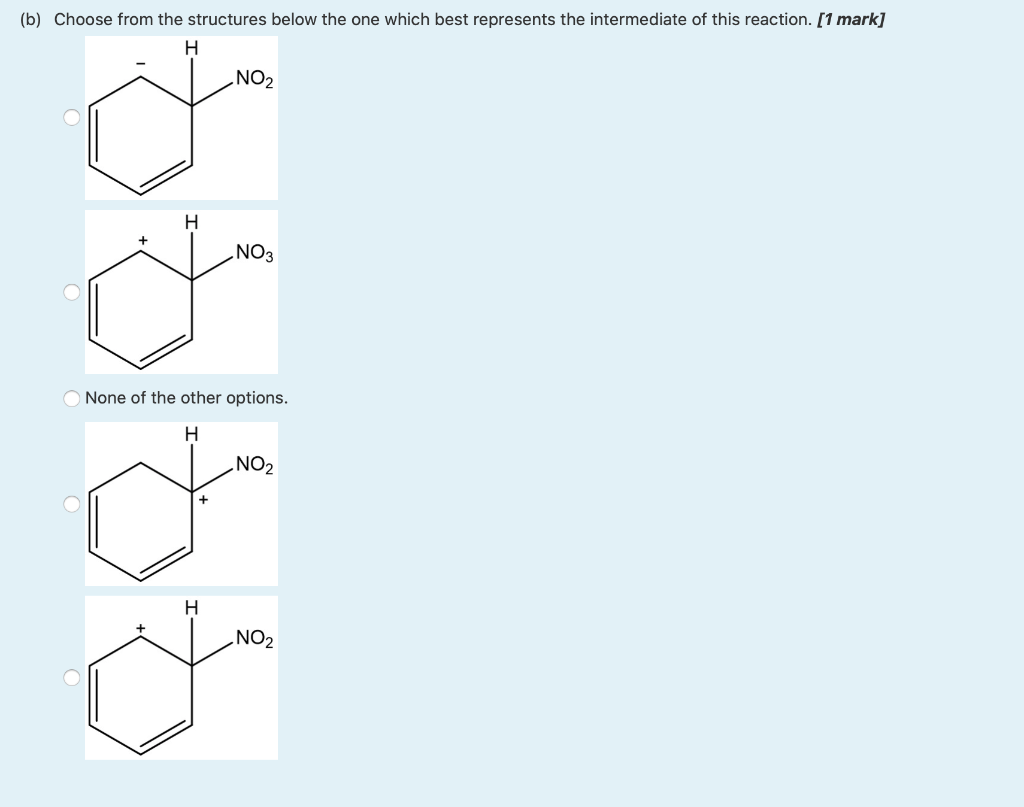
Solved Question 7 5 5 Marks Consider The Following Chegg Com
Comments
Post a Comment